Tag: 510(k) submission
Get Your Device to Market Faster with Expert 510(k) Sub...
shanemason687 Jan 23, 2025 7
The 510k submission is a premarket notification that is filed with the Food and Drug Administration (FDA) for medical devices.
Popular Posts
-
Oil Spill Off California Coast Sends 126,000 Gallons In...
Alex Dec 30, 2024 96
-
Kevin McCarthy’s Plot Bombs As More Republicans Join 1/...
Alex Dec 30, 2024 92
-
Ancient lake in Mars’s Gale crater may have actually be...
Alex Dec 30, 2024 85
-
Check Out The King Of Fighters XV's Isla And K' In Acti...
Alex Dec 30, 2024 78
-
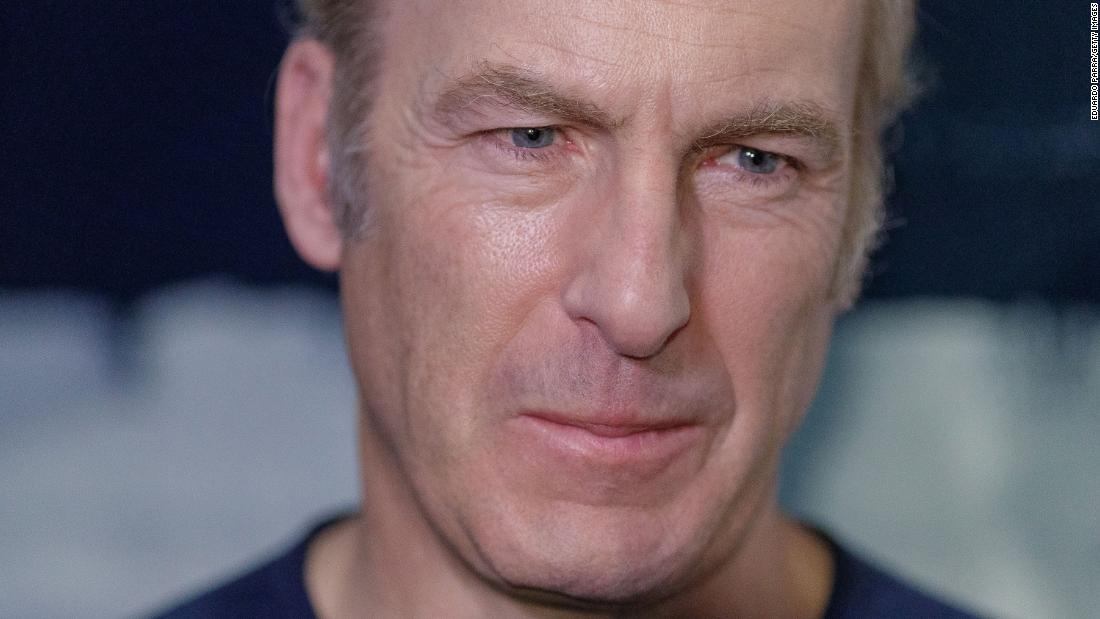
Bob Odenkirk hospitalized after collapsing on set of 'B...
Alex Dec 30, 2024 76
Our Picks
Categories
- Politics(1051)
- Local(541)
- International(833)
- Finance(122)
- Sports(2812)
- Entertainment(858)
- Lifestyle(27)
- Technology(1014)
- Science(701)
- Health(610)
- Crypto(86)
- Press Release(22)
- Legal(201)
- Ecommerce(3)
- Automobile(19)
- Fashion(12)
- Business(41)
- Game Zone(2)
- Travel(4)
- Education(1)
Random Posts
Tags
- custom greaseproof papers
- Knock Knock and Em & Friends promotion
- recruitment companies in dubai
- stacking machinery
- Jftco
- GV GALLERY
- dr shoaib malik
- hire best devops engineer
- Hvac filter suppliers saudi arabia
- Savastan0 CC
- Custom Size Packaging
- DrivingSchoolClayton
- Osteoporosis
- custom food papers
- Corteiz Tracksuit